News about the IVDR
The safety of your patients is important to us
The IVDR, in force since May 26, 2022, represents a turning point in the EU-wide harmonization of patient safety. At the same time, it places high demands on us as manufacturers and on you as a user of in vitro diagnostic medical devices to implement the new regulation. We would like to meet this challenge together with you for the benefit of your patients. It is important to us to certify our portfolio of diagnostic solutions in compliance with the IVDR in due time. Therefore, we would like to keep you informed about our progress with the implementation of the IVDR.
We have been working with a certified quality management system since 2006. Meanwhile, the development, production and sales of our products as well as our technical and application service are subject to a certified quality management system according to ISO 9001:2015 and ISO 13485:2016. In time for May 26, we were able to declare conformity of our non-sterile IVD products in risk class A (e.g. detection reagents) according to IVDR. For our products in risk class C (e.g. antibodies), we have developed a time schedule in close consultation with a notified body. We have been preparing the technical documentation for quite some time to ensure that the majority of in vitro diagnostic medical devices in risk class C will comply with the IVDR as soon as possible within the transitional period, but no later than May 26, 2026.
We would like to take this opportunity to thank you for the trust you have placed in us and we will remain your reliable partner for high-quality in vitro diagnostic medical devices in the future.
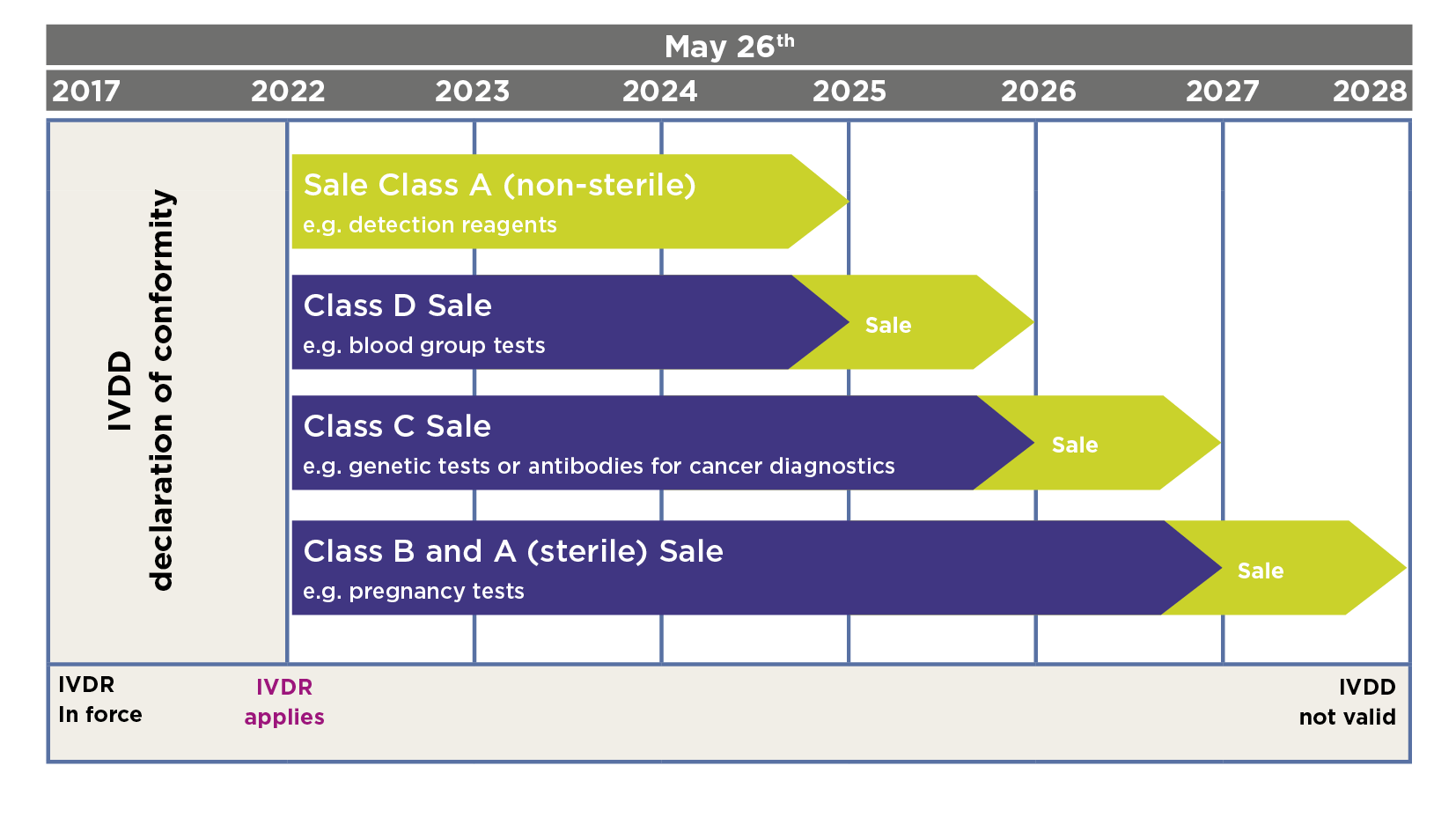
Deadlines for the IVDR:
IVDD-compliant, non-sterile IVD products in risk class A, which have already been placed on the market, may still be sold to end customers until May 25, 2025. Use by end customers is permitted until the expiry date.
Non-sterile IVD products in risk class A produced or newly marketed after May 25, 2022, must already be IVDR-compliant and meet all requirements of the IVDR.
IVD products in risk class C having CE/IVD labelling according to IVDD may still be marketed until May 2026, and products already placed on the market may be sold to end customers until May 2027. Their use is permitted until the expiry date.
As of May 26, 2026, the manufacturer must provide prove of conformity for risk class C in accordance with the IVDR.
Further topics
Qualitative detection of NTRK3 and NTRK1 break apart
Recently, a second ZytoDot ® 2C probe for detecting rearrangements of the NTRK genes has become available: the new ZytoDot ® 2C SPEC NTRK3 Break Apart Probe (C-3079-100). Together with the already existing ZytoDot ® 2C SPEC NTRK1 Break Apart Probe (C-3078-100), a large range of NTRK rearrangements can be covered now. Both probes are designed for the qualitative detection of NTRK3 and NTRK1 rearrangements in FFPE tissue using the ZytoDot ® 2C CISH implementation Kit.

Experts to Experts Program
Workshops & Webinars 2024
