VisionArray®
Detection of HPV, mycobacteria, and fungal pathogens
The CE/IVD-classified VisionArray ® system of our sister company ZytoVision enables sensitive, DNA-chip-based detection of HPV, mycobacteria, and fungal pathogens.
VisionArray® HPV Chip 1.0
Genotyping of human papilloma viruses
We offer the VisionArray® DNA chip system of our sister company ZytoVision for HPV detection. The VisionArray® HPV Chip 1.0 allows simultaneous detection of 41 HPV types with a high sensitivity of up to ≥ 50 copies/reaction. The system is suitable for the examination of DNA from FFPE tissue samples, cervical swabs/brush specimens, as well as from ThinPrep specimens.
Following DNA extraction using standard DNA extraction kits, conserved regions of the HPV L1 gene are PCR amplified using the consensus primers of the VisionArray® HPV PreCise Master Mix. Depending on the HPV type, the amplification reaction creates biotinylated PCR products of a length of approx. 140 bp. Moreover, the assay includes an internal human positive control, HLA-DQA1, as recommended by the WHO.
The biotinylated PCR products are then hybridized on an array with HPV capture sequences. Specifically bound fragments are detected by means of a simple enzymatic reaction using the VisionArray® Detection Kit. The arrays are evaluated by means of a chip scanner and analysis software, which can be handled intuitively.
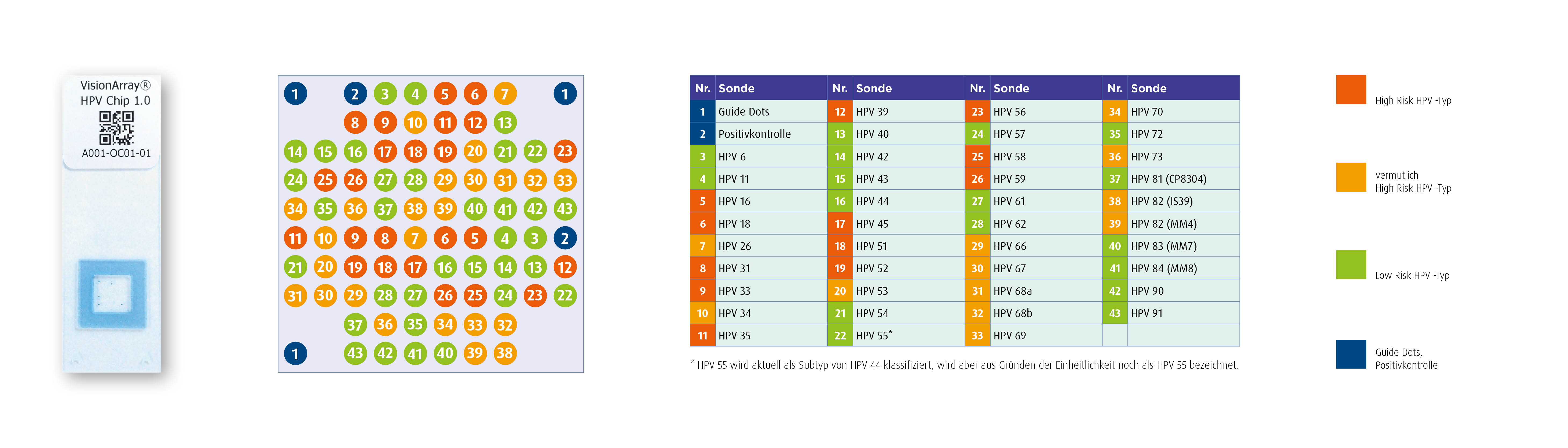
Array-Design
Layout of the VisionArray ® HPV Chip 1.0
Image source: ZytoVision
VisionArray® MYCO Chip 2.0
Genotyping of mycobacteria
The VisionArray® MYCO Chip 2.0 of our sister company ZytoVision is a DNA array for the detection of mycobacteria. The chip detects the M. tuberculosis complex and many clinically relevant nontuberculous mycobacteria types. Both the insertion element IS6110 specific to tuberculous mycobacteria and the 16S-23S rRNA ITS region (internal transcribed spacer) are used to detect M. tuberculosis.
Detection is based on a tried, tested, and robust biochemical detection method. Specific PCR amplicons are generated by means of biotinylated primers and hybridized with the MYCO Chip 2.0. In the process, they bind to complementary capture sequences on the array. Unspecifically bound fragments are removed by stringent washing. Then, a streptavidin-peroxidase-enzyme conjugate is added which converts tetramethylbenzidine (TMB) into a blue stain in a color reaction. The human HLA-DQA1 gene is used as internal positive control, as recommended by the WHO.
The signals are read out by means of an easy-to-use scanner and a connected laptop. An analysis software is available for analyzing the data, which can be handled intuitively. After completion of the PCR reaction, the total time required for array-based detection, scanning of the chip and data analysis is only 60 minutes. Therefore, the VisionArray® MYCO Chip 2.0 provides a highly specific, sensitive and robust system for the detection of the essential clinically relevant mycobacteria types.
Array Design
Layout of the VisionArray ® MYCO Chip 2.0
Image source: ZytoVision

VisionArray® FUNGI Chip 1.0
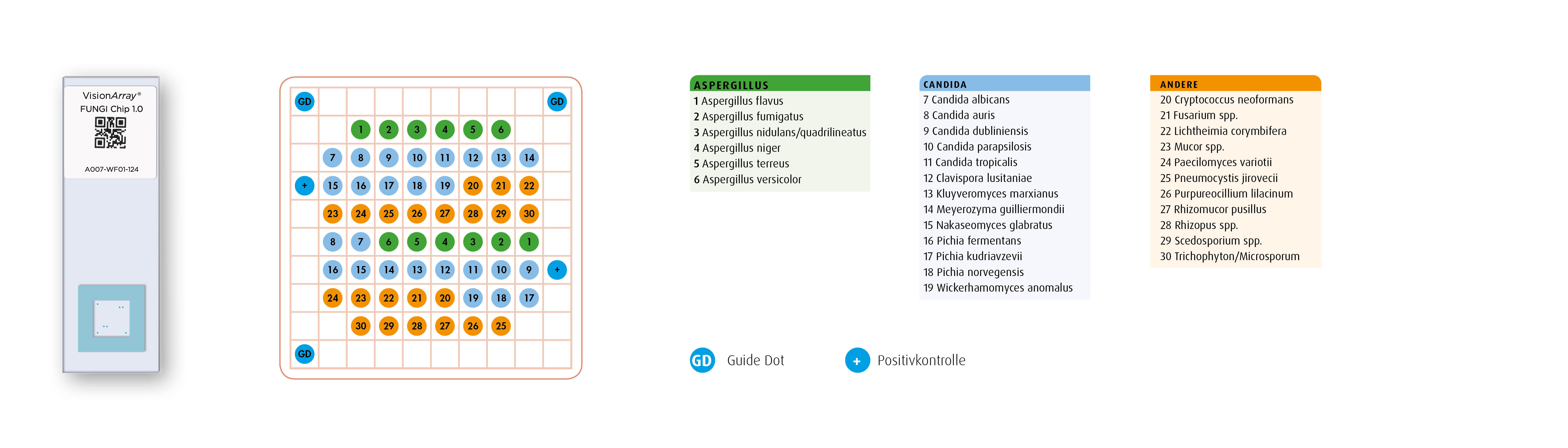
The VisionArray® FUNGI Chip 1.0 represents the most common pathogens of invasive fungal infections. In addition to various Candida and Aspergillus species, Fusarium spp., Mucor spp., P. jirovecii and a number of other species are also represented on the chip. The chip design follows the proven scheme of the VisionArray® series: in addition to the three hybridization controls (guide dots), two dots for a human positive control (HLA-DQA1) are also present, providing evidence for a successful PCR reaction. Two spatially separated dots are present for each species or genus.
After PCR, hybridization and detection occur in as little as one hour, using the same chromogenic detection as for HPV and mycobacteria. All chip types of the VisionArray® series (HPV, MYCO and FUNGI) can therefore be processed and analyzed together.
Array Design
Layout of the VisionArray ® FUNGI Chip 1.0
VisionArray® Chips
| Product | Manufacturer | Amount | Status | Order no. | Info | |
|---|---|---|---|---|---|---|
| VisionArray® HPV Chip 1.0 | ZytoVision GmbH | 10 Arrays (10 Tests) | - | CE/IVD | VA-0001-10 | |
| VisionArray® MYCO Chip 2.0 | ZytoVision GmbH | 10 Arrays (10 Tests) | - | CE/IVD | VA-0005-10 | |
| VisionArray® FUNGI Chip 1.0 | ZytoVision GmbH | 10 Arrays (10 Tests) | - | RUO | VA-0006-10 |
VisionArray ® reagents
| Product | Manufacturer | Amount | Status | Order no. | Info | |
|---|---|---|---|---|---|---|
| VisionArray® HPV PreCise Master Mix | ZytoVision GmbH | 1 Kit (50 Tests) | - | CE/IVD | ES-0007-50 | |
| VisionArray® MYCO PreCise Master Mix 2.0 | ZytoVision GmbH | 1 Kit (50 Tests) | - | CE/IVD | ES-0008-50 | |
| VisionArray® FUNGI PreCise Master Mix 1.0 | ZytoVision GmbH | 1 Kit (50 Tests) | - | RUO | ES-0009-50 | |
| VisionArray® Detection Kit | ZytoVision GmbH | 1 Kit (50 Tests) | - | CE/IVD | VK-0003-50 |
VisionArray ® devices and software
| Product | Manufacturer | Amount | Status | Order no. | Info | |
|---|---|---|---|---|---|---|
| VisionArray® MultiScan Software | ZytoVision GmbH | 1 piece | - | CE/IVD | E-4302-1 | |
| VisionArray® SingleScan Software | ZytoVision GmbH | 1 piece | - | CE/IVD | E-4301-1 |
The VisionArray® SingleScan system:

PC with preinstalled VisionArray Software SingleScan; Scanner 8100; Slide Holder; Hand Scanner; USB-Hub; External Hard Drive; Computer Mouse
Image source: ZytoVision
The VisionArray® MultiScan system:

PC with preinstalled VisionArray Software MultiScan; Scanner V600 Photo; USB-Hub; External Hard Drive; Computer Mouse
Image source: ZytoVision
VisionArray® is a registered trademark of ZytoVision GmbH.